
Why UAE EMS reports are different
Your ePCR isn’t only a clinical narrative. It feeds downstream hospital systems (like NABIDH in Dubai) and must align with DHA and DoH standards, plus the UAE’s federal Personal Data Protection Law (PDPL). Practically, that means you capture specific data elements, time-stamp consistently, record consent and sharing status, and secure any media you collect. Dubai’s Clinical Practice Guidelines (CPGs) and ED element standards explain what hospitals expect to see the moment your patient arrives. Abu Dhabi’s DoH standards add event and MCI reporting requirements for stadiums, festivals, and other mass gatherings. The PDPL sets the privacy guardrails for all health data you handle.
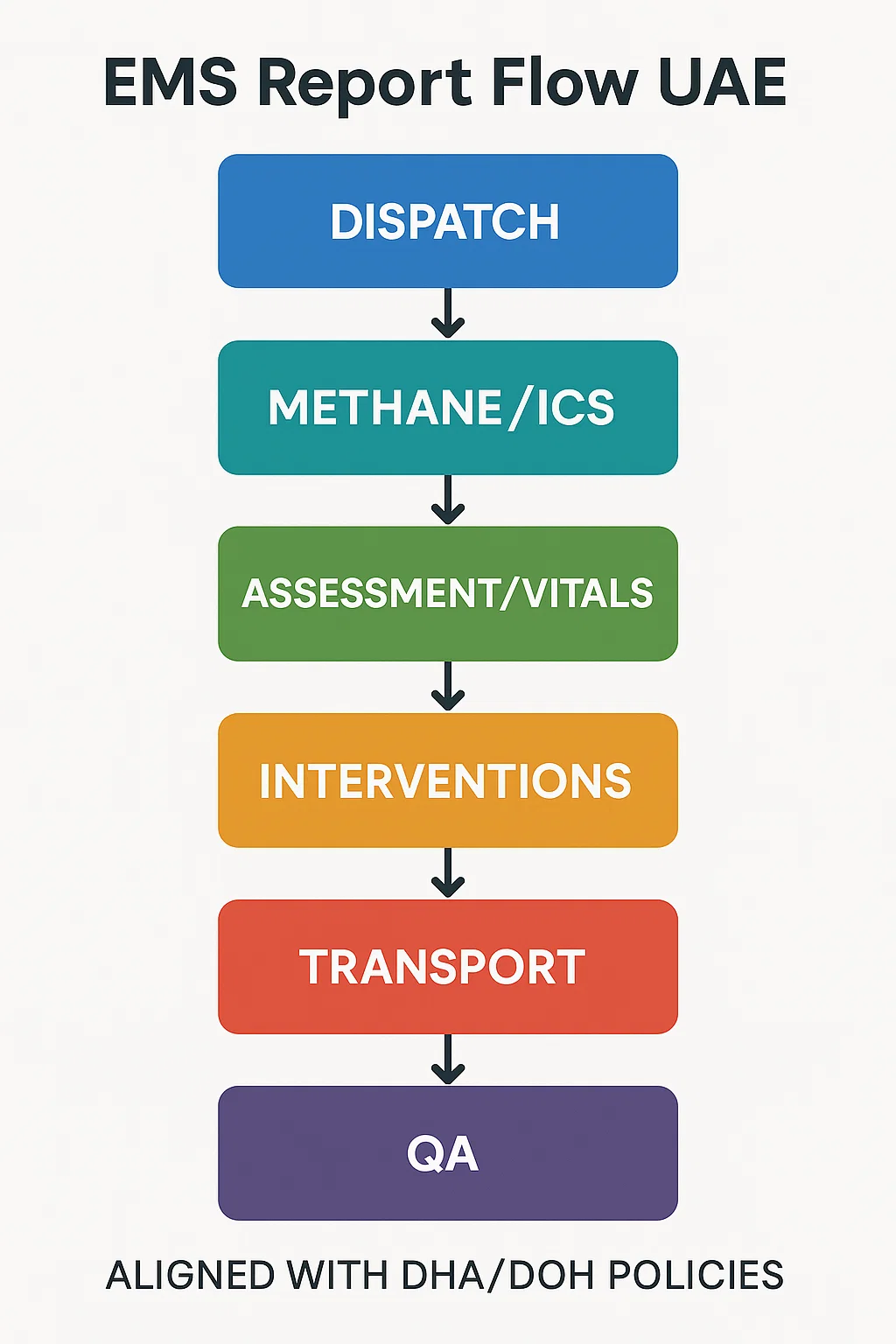
The gold standard flow you can reuse on every call
Think of your report as a clean relay: Dispatch → Scene → Primary data → Interventions → Ongoing assessment → Transport → Handover → Post-run QA. If you log these in order, with crisp times and objective language, your documentation will read like a timeline rather than a memory test.
1) Dispatch and en-route
- Capture who notified you (control center or on-scene services), dispatch code, priority level, and time out/arrival times.
- Note any pre-arrival information that influenced risk (e.g., hazmat, MVC with entrapment).
2) Arrival and scene size-up
- One tight METHANE-style snapshot: Major incident? Exact location, Type of incident, Hazards, Access, Number/casualty types, Emergency services present.
- Safety status, PPE used, and first visual impression of the patient.
3) Primary assessment and vitals
- Airway, breathing, circulation, disability (AVPU/GCS), exposure.
- First full vitals with exact times; pain score required.
- Mechanism of injury or nature of illness, with objective descriptors.
4) Interventions and response
- Every action gets a time stamp, dose/concentration, route, site, and patient response in measurable terms (SpO₂ rose from 89% to 96%; pain 8→4/10).
- Devices and settings: O₂ L/min, CPAP cmH₂O, defib waveforms/energy, 12-lead times/interpretation, glucose values, EtCO₂.
5) Ongoing assessments
- Reassess vitals after each intervention and at set intervals; log trends and any adverse events.
6) Transport and communication
- Decision to transport vs. treat-and-refer with rationale.
- Destination selection factors (specialty center, patient preference, diversion).
- Patch report content and time.
7) Handover and signatures
- Use SBAR content in the narrative; record receiving clinician’s name and handover time.
- Document consents, refusals, or legal exceptions.
8) Post-run quality check
- Scan for red flags: missing times, unmatched interventions and outcomes, absent pain score, loose abbreviations, unsecured photos.
- Lock and submit.
This scene-to-handover structure aligns with Dubai CPGs and DHA ED element expectations, and it slots neatly into DoH event/MCI documentation when you operate in Abu Dhabi.
What the hospital expects to see (and why your times matter)
Emergency departments in Dubai rely on a standardized set of data elements in the ED system fed in part by your ePCR to populate triage, clinical decision support, and continuity of care through NABIDH. That includes patient identifiers, incident times, first recorded vitals, pain score, allergies, medications, past medical history, interventions with times, and clear disposition. If any of these are missing or inconsistent, it creates gaps downstream. Build your template so these fields are hard to skip.
Privacy and consent: write like PDPL is watching
The UAE PDPL governs how personal data is processed, and Dubai’s policies explain how consent and access control work for health information exchanged through NABIDH. In short:
- Capture the legal basis for processing: direct care usually qualifies, but sharing for secondary uses can require explicit consent or another legal condition.
- Note consent status clearly: obtained, refused, or not possible (e.g., unconscious) and why.
- Record minimum necessary details, secure any attachments (photos, audio), and follow your service’s retention rules.
These points map to DHA’s consent and access standards and the PDPL’s overarching obligations.
The anatomy of a high-quality narrative
Aim for an objective, chronological story with three quick lenses: what you found, what you did, how the patient changed.
Good
“Found adult male seated, speaking full sentences, clutching left chest. Initial vitals 142/88, HR 112, RR 22, SpO₂ 94% RA, pain 8/10. ASA 300 mg chewed 19:07 after allergy check. 12-lead 19:10: ST elevation V2–V4; patch to ED at 19:12. O₂ 4 L/min NC, pain ↓ to 4/10 at 19:18. Transported priority, arrival 19:26. SBAR handover to Dr. Khan 19:28.”
Weak
“Male c/o chest pain. Given meds. Transported to ED.”
The first one is defendable, auditable, and clinically useful. The second one makes your receiving team and your QA officer work too hard.
Table: Minimum data to nail every time
| Section | Must-have elements | Notes |
|---|---|---|
| Dispatch & Times | Time out, on-scene, patient contact, depart scene, arrive ED | Use device sync to keep clocks aligned |
| Patient ID | Full name, ID or MRN if available, age/DOB, sex | Verify spelling; align with hospital ID at handover |
| Clinical | Chief complaint, mechanism/nature, allergies, meds, PMH | Avoid vague complaints without descriptors |
| Vitals | Baseline vitals with times; pain score; GCS/AVPU | Repeat after interventions and before handover |
| Interventions | Drug, dose, route, time, response; devices/settings | Include device serials if policy requires |
| Media | 12-lead time and interpretation; photos if authorized | Store securely; reference consent status |
| Transport | Destination, mode/priority, rationale, en-route changes | Record diversion or specialty center decisions |
| Handover | SBAR content, receiving clinician, handover time | Confirm ED has your identifiers and times |
These categories reflect the DHA ED element standard and common ePCR buildouts across the Emirates.
Checklist: your 90-second pre-submit scan
- All times present and in order
- First and last vitals, plus post-intervention vitals
- Pain score recorded at least once (and updated if treated)
- Specific drug/dose/route/time with response
- Clear consent/refusal status; media secured
- SBAR handover recorded with name/time
- Spelling of patient ID and location verified
- No unexplained gaps or subjective judgments
Pro tip box #1: Write like the body cam is on
Stick to observable facts. Replace “drunk patient” with “smell of alcohol on breath, unsteady gait, slurred speech, admits to 6 beers.” Objective phrasing protects you under QA and legal scrutiny and fits policy language for data quality and subject-of-care rights.
How to document refusal correctly
Refusal notes are where crews most often get burned. Your steps:
- Assess decision-making capacity: oriented, understands risks/benefits/alternatives, able to paraphrase back.
- Offer alternatives (GP, urgent care, family supervision).
- Record a specific risk statement you explained and the patient’s response.
- Capture vitals, document any treatment provided, and log your advice to return or call if worse.
- Obtain signature if possible; if not, document why and have a witness sign.
These elements align with consent/rights language used by DHA and general EMS risk practice.
Pro tip box #2: Times are treatment
In STEMI, stroke, sepsis, and trauma, times become the treatment door-to-balloon, door-to-needle, trauma activation. Your report’s time stamps can accelerate pathways if they’re clear, consistent, and visible at triage. The DHA ED standard exists to make those pathways faster and safer across the system.
Event medicine and MCI reporting in Abu Dhabi
For planned events and mass gatherings, DoH standards expect defined medical coverage, incident logging, patient-tracking, and reporting back to the organizer and the regulator. In MCIs, documentation shifts to triage counts, resource/treatment sectors, patient tracking IDs, and transfer destinations. Your ePCR still matters only now it’s one record in a large, auditable picture. Build your incident narrative around METHANE updates, sector assignments, and casualty flow.
The SBAR handover you should copy-paste
- Situation: “Adult with crushing chest pain; 40 minutes since onset.”
- Background: “HTN, smoker; aspirin given; no known allergies.”
- Assessment: “ST elevation V2–V4; vitals currently 128/84, HR 98, SpO₂ 97% on 4 L.”
- Recommendation: “Cardiology activation started; request immediate ECG review.”
Use SBAR wording in your narrative so what you said at the bedside matches what’s in the report.
Documentation examples you can borrow
Trauma, road traffic collision
“Arrived 17:42 to single-vehicle RTC, driver restrained, airbag deployed. METHANE: No MCI. Scene safe, access via southbound service lane. Adult male, alert, seated roadside. Primary: patent airway; RR 24 labored; radial pulses present; minor bleeding from scalp. Pain 7/10 occipital. Vitals 17:46: 136/86, HR 108, SpO₂ 93% RA, GCS 15. Supplemental O₂ 4 L/min NC 17:48 → SpO₂ 97% 17:53. C-spine precautions, wound dressed with pressure. No neuro deficit. Transported 17:56 to trauma center per protocol. 18:10 SBAR to Dr. Ahmed; handover complete 18:12.”
Medical, hyperglycemia
“Call 22:08 for ‘weakness.’ Adult female, ambulatory, speaking full sentences. Fingerstick 22:15 = 27.8 mmol/L. IV cannula 20G left forearm 22:18; 0.9% NaCl 250 mL started. No chest pain; clear lungs; mild dehydration. Re-check 22:29 = 22.0 mmol/L; patient more alert. Transported non-urgent to nearest ED; SBAR 22:44 to charge nurse.”
Common pitfalls in UAE reports
- Missing consent status → Add a dedicated field in your template. “Consent: implied for direct care; no secondary use without consent. No photos taken.”
- Vague MOI/NOI → Replace “fall” with “fall from ~1.5 m ladder, landed on right side, no LOC per spouse.”
- No post-intervention vitals → Commit to “intervention + 5” re-check habit.
- Unsecured media → Store only in approved ePCR, never on personal devices; note “stored per policy.”
- Abbreviations without expansion → Write first mention in full: “Continuous Positive Airway Pressure (CPAP).”
Building your personal template
Create boilerplate headings in your ePCR or a pocket card:
- Dispatch/Times
- METHANE & Scene
- Primary Assessment + First Vitals
- Interventions (time/dose/route/response)
- Ongoing Vitals/Trend
- Transport & Patch
- SBAR & Handover
- Consent/Privacy Note
- QA Check (8-point list)
These headings mirror how EDs consume your data, especially where Dubai’s ED data elements and NABIDH exchange require completeness.
Quick QA rubric you can apply across your fleet
Score each report 0–2 in these five domains (max 10):
- Completeness: All mandatory elements present (IDs, times, vitals, pain score).
- Clinical clarity: Primary complaint and MOI/NOI well described; assessments make sense.
- Intervention fidelity: Drug/device parameters and responses documented.
- Continuity: SBAR handover recorded; receiving clinician and time logged.
- Compliance: Consent/privacy noted; no unsecured media; abbreviations appropriate.
Attach targeted feedback: “Great vitals and post-intervention notes. Next time, include pain score before and after fentanyl.”
Frequently asked questions
Do I need explicit consent to share my ePCR with the hospital?
Not for direct care. DHA’s policy notes consent is needed when sharing for reasons beyond direct care unless another legal basis applies. Record consent status in your report.
What if the patient refuses transport but wants treatment?
Treat within scope, confirm capacity, explain risks, document the conversation, and record the refusal with a witness if possible. This aligns with consent/access expectations and protects your crew.
Are photos ever okay?
Only where your service policy allows and storage is within the approved system. Always note consent and purpose; avoid capturing bystanders or identifiers. DHA confidentiality policy and PDPL both expect strict controls.
What makes my report “court-ready”?
Clear chronology, objective wording, complete times and identifiers, explicit consent/refusal notes, and documented responses to treatment. That’s the same content the ED and regulators rely on for quality and safety.

Alex Smith, a seasoned medical technician with 15 years in ambulance services, writes crucial first-aid tips and emergency care insights on arescuer.com.
