The Fire Chemistry in Fire Start to End
OBJECTIVES:
- Upon completion of this lesson, you will be able to:
- Define fire (combustion process) and the triangle of fire.
- Describe the radiation feedback, the chain reaction and fire tetrahedron.
- Describe the products of fire and their characteristics
1. Definition of Fire:
Combustion is a chemical reaction or series of chemical reactions in which fuel combines chemically with an oxidizing agent, as a result of which a sufficient quantity of energy in form of heat, flame and light etc. is released.
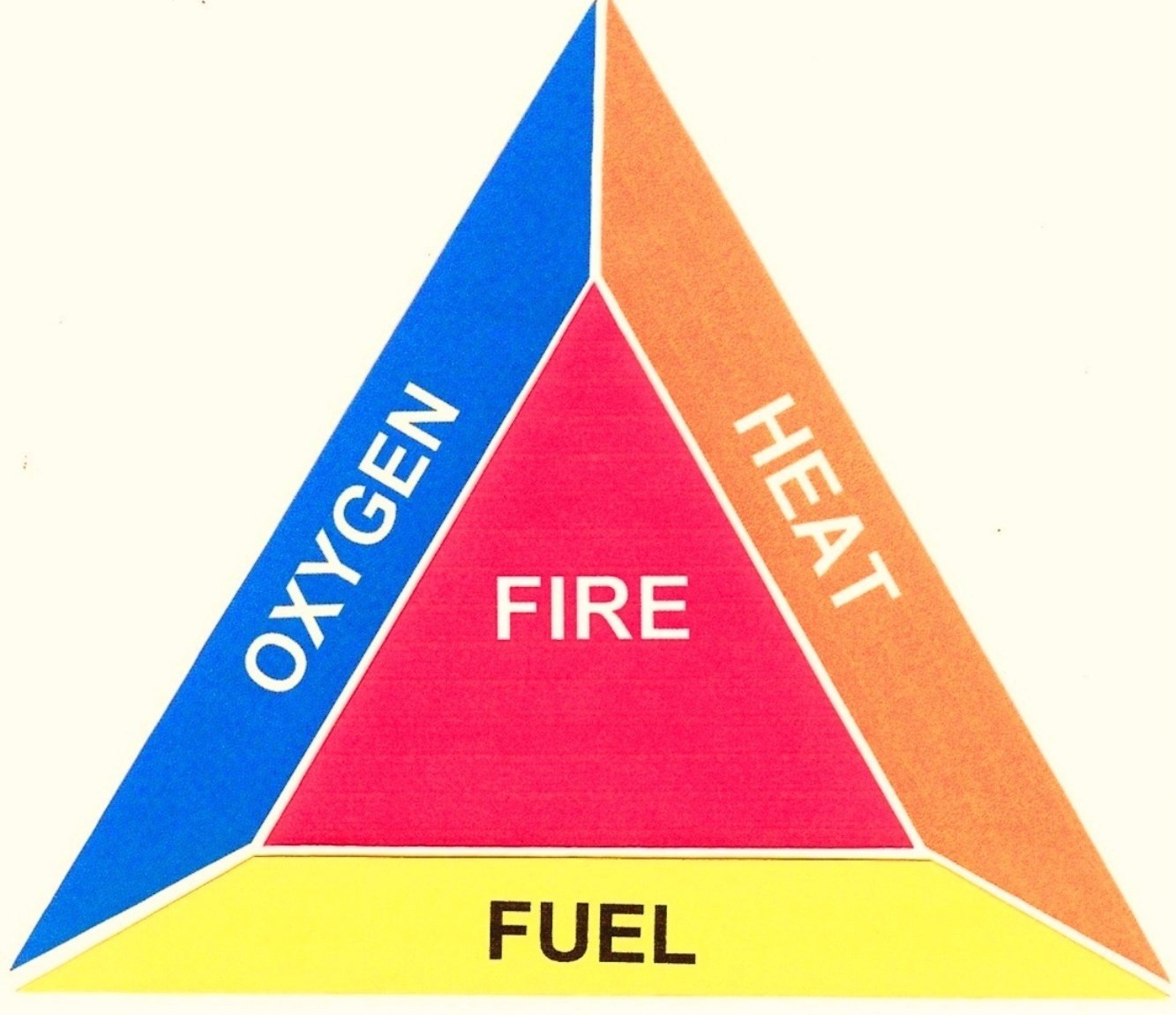
1.1. What is Triangle of Fire:
According to the law of triangle of combustion, following three basic things are necessary for creating a fire.
- Fuel
- Oxygen
- Heat or Temperature
A Model fire Triangle
2. Radiation Feedback:
The part of heat which radiates back to the seat of fire and further propagates the fire
2.1. The Chain Reaction:
The Chain reaction is a process in which many fire triangles ‘combines each other to light the fire continuously.
Burning is the rapid oxidation of millions of vapor molecules, during this process energy is released as heat and light which is pure energy or radiant heat, it radiates or travels in all directions thus part of this moves to the seat of fire or to the burning fuel in fire fighting. It is called Radiation Feedback. In the same time air is drawn into the area, where the flame and vapor meets.
The result is that newly formed vapors begin to burn. The flame increase, thus starting chain reaction. As long as there is plenty of fuel, fire continues to grown.
2.2 The Fire Tetrahedron:
Model Fire Tetrahedron Diagram
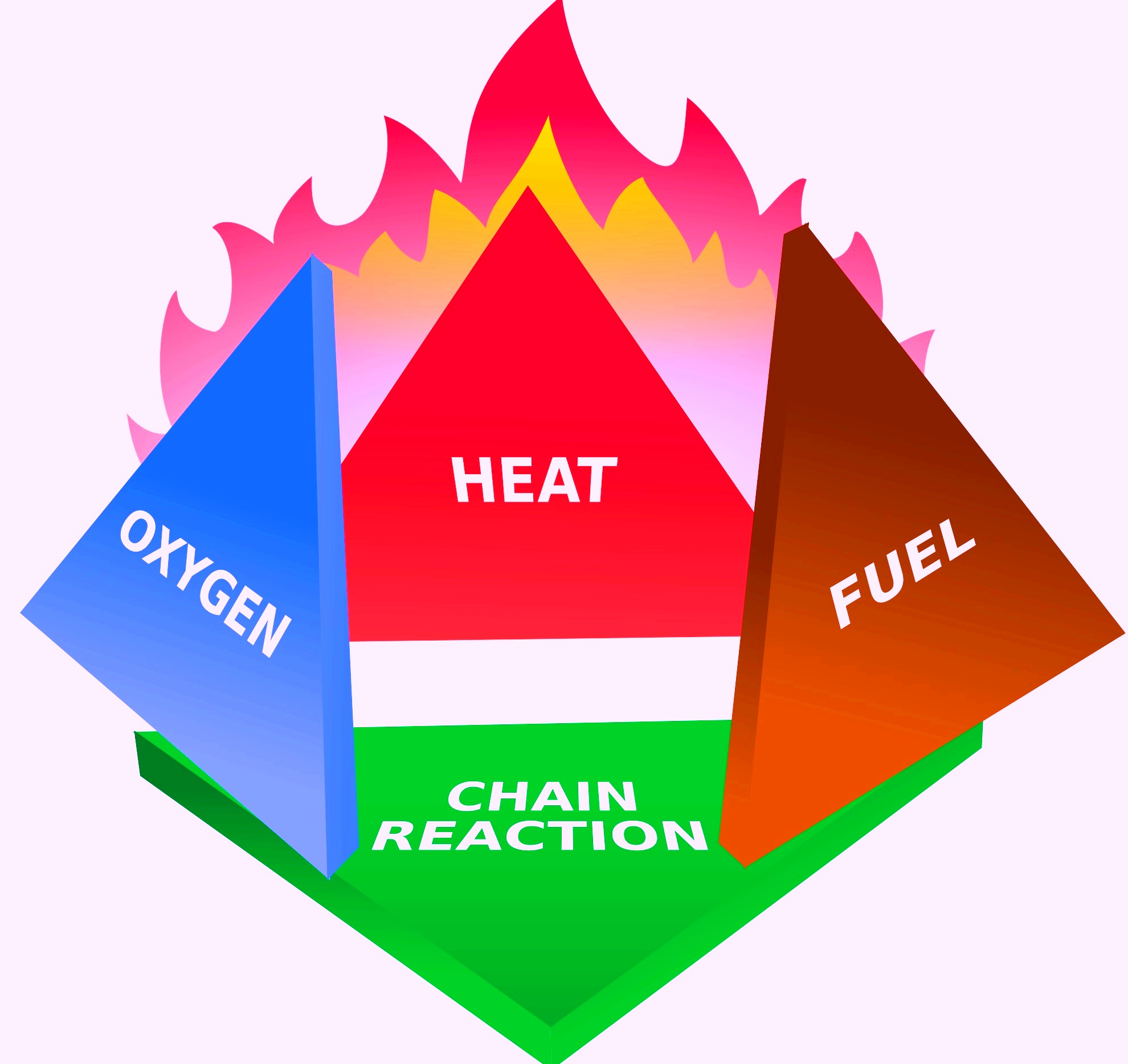
Contents of Fire tetrahedron:
- Fuel
- Heat
- Oxygen
- Chain Reaction
3. Products of Fire:
The Major fire products are:
Smoke.
Fire gases.
Flame.
Heat.
Ash.
Light.
3.1 Smoke:
“Smoke is colloidal dispersion of a solid In a gas”.
It consists of:
Particle (burnt, semi burnt, un-burnt).
Water vapors.
Toxics & combustible gases.
Smoke is generally a visible sign of fire.
3.1.1 Effects of Smoke:
Smoke is major cause of fire spread.
Presence of smoke creates less visibility.
Inhalation can cause severe injuries.
3.1.2 Types of smoke:
Wooden smoke (unburned carbon particles).
Metallic smoke (unburned metal ore particles).
Chemical smoke (unburned particles of chemical).
Cigarette smoke (nicotine particles).
3.2 Fire Gases:
The effects of toxic gases will depend on:
Time of exposure.
Concentration of gases in air.
Physical condition of individual.
There are usually several gases present during fire such as:
Carbon dioxide carbon monoxide, hydrogen sulfide,
Sulpher dioxide ammonia, hydrogen cyanide,
Hydrogen chloride, nitrogen dioxide and phosgene.
3.3 Flame:
Flame, a result of combustion, produces special risks like:
Fire due to direct contact with flame.
Emits light.
Release of sufficient heat.
3.4 Heat:
Heat is also generated in the chemical reaction and has its own affects like:
Further propagation of fire.
Burns caused to the fire fighter.
3.5 Ash
This is The solid that remains of fires and does not have further process to make fire increase.
3.6 Light
Light is electromagnetic radiation released during the fire combustion process.

John Doe, a seasoned firefighter, shares his vast knowledge of fire safety and emergency preparedness at arescuer.com, aiming to empower and educate
